Background: The coronavirus disease pandemic of 2019 (COVID-19) has been associated with coagulopathy and an increased rate of thrombosis in adults. Medical practitioners have been prompted to consider prophylactic anticoagulation in special populations diagnosed with COVID-19. Patients with sickle cell disease (SCD) are predisposed to a hypercoagulable state. Despite the concern for development of venous thromboembolism (VTE) in these patients, there are no standardized guidelines for routine thromboprophylaxis in either adults or children with SCD. Thus, VTE management options are often extrapolated from guidelines for the general population.
Methods: A cross-sectional electronic survey was distributed to pediatric and adult hematology oncology practitioners through 7 SCD specific interest groups. Pediatric and adult practitioners were defined as those who medically manage patients 0-21 years of age and >21 years of age respectively. We examined responses to survey questions focused on routine thromboprophylaxis practices in children and adults with SCD prior to the pandemic and in patients with SCD admitted with COVID-19. Chi-square analyses or Fisher's exact tests for small samples were used to compare proportions as needed.
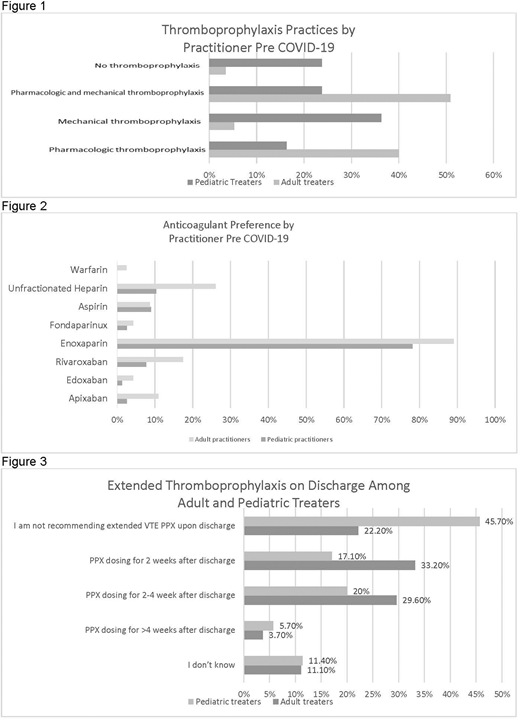
Results: The survey was distributed to approximately 2,550 providers. Of 93 total responses, 14% (N=13) only treat patients >21yo; 38.7% (N=36) only treat patients 0-21yo and 47.3% (N=44) treat both. Nearly all adult practitioners (96.6%) would recommend pharmacologic prophylaxis, mechanical prophylaxis or both for hospitalized adults, but only 76% of pediatric treaters would recommend any prophylaxis (PPX) in hospitalized children (p<0.0001, Figure 1). Only 16% would recommend pharmacologic PPX only for patients 0-21yo, with 36% preferring non-pharmacologic only methods and 24% preferring both forms. In contrast, for the >21yo patients, 51% preferred to use both pharmacologic and non-pharmacologic PPX, 25% preferred pharmacologic alone, with just 5.3% preferring non-pharmacologic PPX only. Enoxaparin was the most frequently used anticoagulant among both pediatric and adult practitioners [78% vs 89% respectively] (Figure 2). Direct oral anticoagulants (DOACS) were infrequently recommended (3.8% for SCD children and 10.9% for adults); of these, rivaroxaban was used most often. The most common indication for starting thromboprophylaxis for both 0-21yo and >21yo patients was history of prior VTE [81% and 81% respectively], followed by hip replacement [57% and 75% respectively].
In patients admitted with COVID-19, prophylactic anticoagulation was recommended for adults by 94% of treaters. There was a significant increase in the use of prophylactic anticoagulation in children with COVID-19 vs. those without (84% vs. 40%; p=.0001). Figure 3 shows extended thromboprophylaxis practices upon discharge for COVID related admissions. Almost half of respondents (46%) would not recommend any thromboprophylaxis for children on discharge; 37% would recommend either 2-4 weeks or >4weeks of post-discharge PPX. The majority of adult providers would recommend discharge thromboprophylaxis for adults with 2 weeks post discharge (33%), and 2-4 weeks (30%) being the most common regimens.
Conclusion:
This pilot survey describes the thromboprophylaxis practices used for adult and pediatric SCD patients by specialty practitioners. In general, practitioners were likely to prescribe pharmacologic thromboprophylaxis for adults while mechanical PPX was preferred for children. However, in the high-risk setting of COVID-19 infection, pediatric practitioners would modify their practice to include pharmacologic thromboprophylaxis. Interestingly, despite the long-term availability of direct oral anticoagulants in adults, and recent completion of pediatric studies, the most commonly used pharmacologic agent pre-COVID in adults and children was enoxaparin. Of note, this survey was conducted prior to release of the International Society on Thrombosis and Haemostasis guidelines regarding discharge PPX in COVID-19. (Spyropoulos AC, J Thromb Haemost, 2020)
These results highlight the influence of COVID-19 on the use of pharmacologic thromboprophylaxis, specifically in the pediatric population. Due to frequent hospitalization, studies are needed to guide decision making surrounding VTE PPX for the adult and pediatric inpatient SCD population.
Davila:Spire Learning: Speakers Bureau; ATHN: Other: Grant Funding. Minniti:Bluebird bio: Consultancy, Research Funding; TauTona: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Emmaus: Consultancy, Research Funding; CLS Bering: Consultancy; Novartis: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding. Desai:Pfizer, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; GBT, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ironwood Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees; Rockpointe Continuing Medical Education Company: Consultancy. O'Brien:Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Enoxaparin used as a form of thromboprophylaxis in children.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal